-
Controlling singlet–triplet splitting in carbazole–oxadiazole based bipolar phosphorescent host materials
P. Kautny, Z. Wu, B. Stöger, A. Tissot, E. Horkel, J. Chen, D. Ma, H. Hagemann, J. Fröhlich and D. Lumpi
Organic electronics, 17 (2015), p216-228


DOI:10.1016/j.orgel.2014.11.027 | unige:44949 | Article HTML | Article PDF | Supporting Info

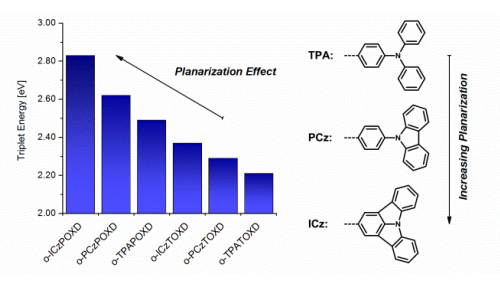
A rational molecular design strategy for carbazole–oxadiazole based bipolar host materials was developed to improve the device efficiency of blue phosphorescent organic light-emitting diodes (PHOLED). Steric effects of strategically placed methyl groups led to an increase of triplet energies (o-2MPCzPOXD: 2.66 eV and o-3MPCzPOXD: 2.73 eV versus the initial host material o-PczPOXD: 2.62 eV) while less pronouncedly affecting singlet energies and, therefore, retaining low driving voltages, high power efficiencies and remarkably low efficiency roll-offs in PHOLEDs. The maximum quantum efficiencies (EQE) for blue devices (FIrpic) were significantly raised for o-2MPCzPOXD (13.6%) and o-3MPCzPOXD (11.5%) versus o-PCzPOXD (9.0%) although yielding comparable values for green devices (Ir(ppy)3; 12.9% and 15.4% versus 13.2%). Supported by theoretical calculations a structure–property relationship was established from photo-physical properties, PHOLED performance measurements and structural characterization from single crystal data.